As health systems increasingly depend on vendors to provide essential medical equipment rental and service, one concerning quality gap has emerged: a lack of regulatory oversight of those service providers. Part 1 of a 4-part series exploring the gaps surrounding medical equipment quality.
A Focus on the Details
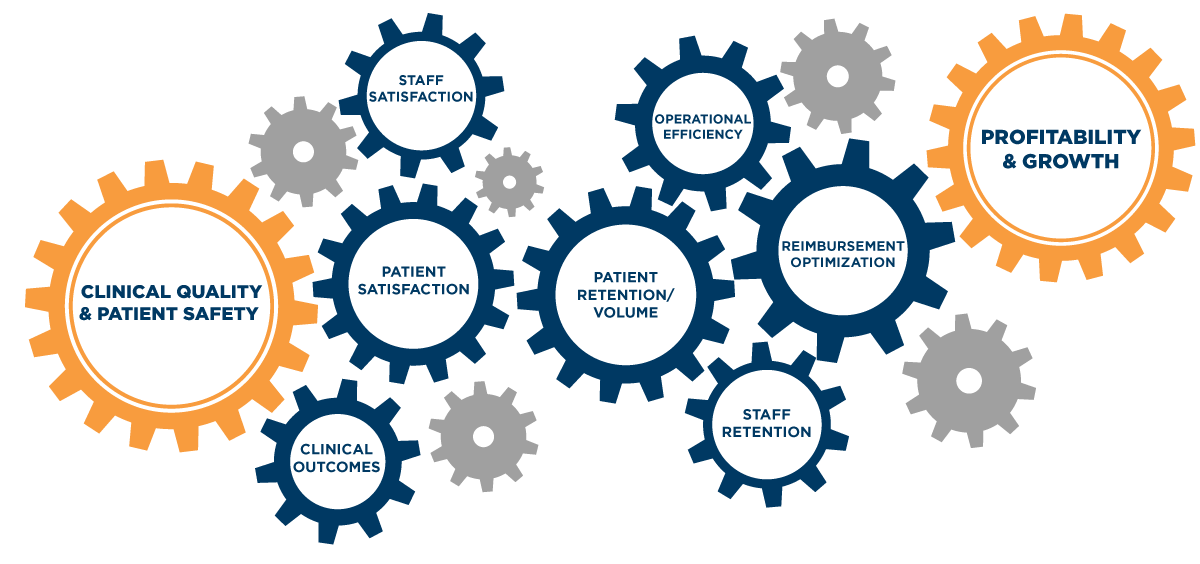
Healthcare has always been detail-focused. It has to be. It’s one of those rare fields where little things can make the difference between life and death — where “quality” and “safety” are synonymous. But with the increasing demands of value-based care — and the rising expectations of patients as consumers — the small factors that influence clinical quality and patient safety now fuel a larger business-outcomes engine, driving critical outcomes ranging from staff satisfaction and patient retention to profitability and growth.
In this new environment, there’s simply no room for risk; there’s no such thing as a harmless shortcut. Yet several concerning quality gaps have emerged — and many are sitting right at your patients’ bedsides and operating tables.
Over the next few weeks, we will dive into some of the patient safety gaps surrounding medical equipment management to better understand factors negatively affecting risk, and how to protect your patients and business — in four distinct parts:
- The Quality Engine: How the Vendors you choose impact your facility (Today’s Blog)
- Why “ISO-certified” Isn’t Enough: The Difference Between ISO 13485:2016 and ISO 9001:2015
- Understanding ANSI: What New Standards Mean for Your Laser Operators
- Evaluating Vendor Quality: Best Practices for Closing the Quality Gap
The Quality Engine
Every aspect of a healthcare organization — from clinical decision-making to the condition of hallway floors — is under increasing scrutiny. Whether due to the Affordable Care Act’s broad-sweeping initiatives, or based on individual regulatory updates, the margin for error is shrinking and the penalties are increasing. Hospitals need to be able to quickly adapt to evolving regulatory standards and guidelines that govern clinical quality and patient safety. A commitment to quality is no longer enough. Quality needs to be embedded into the operations of the hospital, because business objectives are now tied back to it. In short, clinical quality fuels the outcomes you care about most.
Quality Case Study: Medical Equipment Readiness
Imagine a nurse needs an infusion pump. If the nurse can quickly find a device that’s clean, patient-ready and has been functionality tested, there’s no delay in care to the patient. Apply this across all your movable medical equipment and you can see the quality engine in motion: Smart workflows improve medical equipment availability and prevent care delays. This not only reduces excess cost, it also enhances patient experiences and improves staff satisfaction. This leads to improved clinical outcomes, which maximize reimbursements and expand margins. Higher margins drive profitability and unlock growth potential. All of these benefits can be gained by having a single infusion pump readily available for care.
However, those same small cogs, if interrupted, can also cause serious breakdowns in the quality engine. If a device doesn’t work or is not available when needed, care is delayed — frustrating both patients and staff. These delays decrease treatment efficiencies and require staff to spend time hunting for a patient-ready device. Often, additional equipment is rented or purchased to patch this perceived gap in available equipment — increasing costs and cutting into margins.
Medical equipment rental providers and service providers are not regulated by the FDA, nor are they required by law to adhere to OEM quality standards.
At the same time, patient and staff frustration drive rippling effects — decreased staff productivity, higher turnover costs and lower patient retention stemming from a poor care experience. Not to mention the biggest risk: that a care delay (or faulty equipment) will lead to a patient safety incident.
Key Challenge: Building (and Maintaining) a Reliable Quality Engine
As the complexity of modern healthcare delivery rapidly increases — more technology, more advanced care, and more demanding regulations — there are significantly more small cogs that play a critical role in the quality engine. Even the most cutting-edge organizations struggle to consistently manage all of these small factors. Additionally, attempting to manage all of this increasing complexity isn’t in their financial or clinical best interests — it diverts attention and resources from the actual hands-on delivery of exceptional patient care.
This leads more healthcare organizations to lean on third-party service partners to handle peripheral aspects of the quality engine. For example, to address medical device challenges like the infusion pump example discussed above, hospitals and health systems increasingly leverage medical device rental and service vendors to ensure state-of-the-art, patient-ready equipment is available when and where it’s needed.
As healthcare organizations lean more heavily on third-party service providers, they need to be prepared to ask the right questions on their quality and patient safety practices.
The Hidden Risk Most Providers Miss
As health systems increasingly depend on vendors to provide essential medical equipment rental and service, one concerning quality gap has emerged: medical equipment rental providers and service providers are not regulated by the FDA, nor are they required by law to adhere to OEM quality standards. This is true even for critical considerations like installing OEM-approved replacement parts for integral components within a device, or ensuring the technician servicing the equipment has received the appropriate training.
This means critical life-saving medical devices may not be maintained to the standards that you expect — which could lead to poor performance or even equipment failures that threaten patient safety. These quality shortcomings also create jams in the quality engine, with problematic impacts down the line — from patient experience and staff satisfaction, to operational efficiency, and profitability.
Imagine a rental vendor drops off a brand-new device that is covered under warranty. No quality risk there, right? Wrong. Many vendors fail to communicate the service and support plan that will follow a device across its useful life. Specifically, how will the device be cleaned, tested and maintained on an ongoing basis to ensure it continues to operate at an optimal, safe level for patients? Since there is no governance of third-party providers, vendors can choose not to follow the most current standards for quality and patient safety, which can introduce a higher level of risk to your patients and organization.
The Key Question: How Do You Evaluate Vendor Quality?
As critical cogs in the quality engine fall in the hands of third-party vendors, the question becomes: how can your organization evaluate, monitor and ensure vendor quality?
In our next chapter, we will dive into the differences in ISO certification standards — including the growing gap between the medical device specific ISO 13485:2016 and the generic ISO 9001:2015 – and why that difference could put your patients and bottom line at risk.
Read the Full Report Today
Don’t want to wait? You can download the full report today — from the nuances in ISO standards and how they impact patient safety, to tips for how to choose vendors that best support the delivery of quality clinical outcomes and exceptional patient experiences.
Visit our Resource Center to discover more, or return to the blog menu.